
Physics, 24.07.2019 08:30 bossninja97588
Asample of 20.0 moles of a monatomic ideal gas (γ = 1.67) undergoes an adiabatic process. the initial pressure is and the initial temperature is the final temperature of the gas is what is the final volume of the gas? let the ideal-gas constant r = 8.314 j/(mol • k). a) 350 l b) 270 l c) 190 l d) 230 l

Answers: 1
Another question on Physics


Physics, 23.06.2019 02:30
Substances that assume the shape of their container, but do not have a definite size are called
Answers: 2

Physics, 23.06.2019 05:30
Aman jogs at a velocity of 2.5 m/s west for 1,200 s. he then walks at a velocity 1.0 m/s east for 500 s and then stops to rest . what is the displacement of man when he stops to rest?
Answers: 3

Physics, 23.06.2019 08:00
Use henry's law and the solubilities given below to calculate the total volume of nitrogen and oxygen gas that should bubble out of 1.7 l of water upon warming from 25 ˚c to 50 ˚c. assume that the water is initially saturated with nitrogen and oxygen gas at 25 ˚c and a total pressure of 1.0 atm. assume that the gas bubbles out at a temperature of 50 ˚c. the solubility of oxygen gas at 50 ˚c is 27.8 mg/l at an oxygen pressure of 1.00 atm. the solubility of nitrogen gas at 50 ˚c is 14.6 mg/l at a nitrogen pressure of 1.00 atm. assume that the air above the water contains an oxygen partial pressure of 0.21 atm and a nitrogen partial pressure of 0.78 atm.
Answers: 2
You know the right answer?
Asample of 20.0 moles of a monatomic ideal gas (γ = 1.67) undergoes an adiabatic process. the initia...
Questions

Social Studies, 24.01.2020 21:31


Mathematics, 24.01.2020 21:31



English, 24.01.2020 21:31

English, 24.01.2020 21:31



Biology, 24.01.2020 21:31

Physics, 24.01.2020 21:31

Biology, 24.01.2020 21:31



Physics, 24.01.2020 21:31


Biology, 24.01.2020 21:31

History, 24.01.2020 21:31


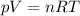
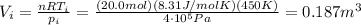
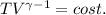
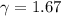 for a monoatomic gas as in this exercise. The previous relationship can be also written as
for a monoatomic gas as in this exercise. The previous relationship can be also written as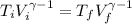
![V_f = V_i \sqrt[\gamma-1]{ \frac{T_i}{T_f} }=(0.187 m^3) \sqrt[0.67]{ \frac{450 K}{320 K} }=0.310 m^3 = 310 L](/tpl/images/0126/6187/97c57.png)


