Physics, 08.10.2019 08:10 ewymer3901
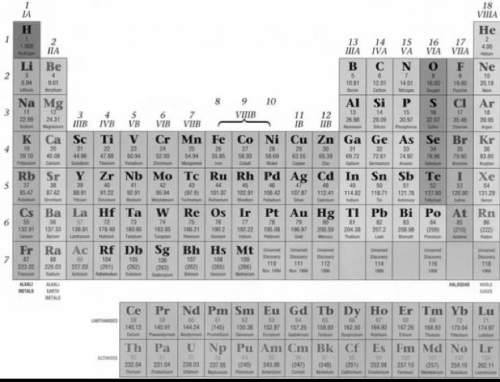
The oxidation number of all metals on the periodic table have a positive (+) charge. which of these best explains why?

Answers: 2
Another question on Physics

Physics, 22.06.2019 02:30
Agas initially at p1 = 1 bar and occupying a volume of 0.5 liter is compressed within a piston–cylinder assembly to a final pressure p2 = 4 bar. (a) if the relationship between pressure and volume during the compression is pv = constant, determine the volume, in liters, at a pressure of 3 bar. (b) repeat for a linear pressure–volume relationship between the same end states. reference
Answers: 1

Physics, 22.06.2019 04:30
Light from the sun reaches the earth in 8.3 minutes. the velocity is 3.00 x 10^8 m/s. how far is earth from the sun? i know how to get to d = (3 x 10^8 m/s)(498 sec). i just don't know how to get from there to the answer being 1.5 x 10^11 m.
Answers: 3

Physics, 22.06.2019 05:30
Imagine that someone pushes one marble toward a motionless marble. would there still be action-reaction forces involved in the collision? how might the marbles’ motions be changed? ?
Answers: 1

Physics, 22.06.2019 11:40
Imagine that you have two balloons (or, better yet, actually inflate two balloons, if possible). create static electricity around one of the balloons by rubbing it against your hair or your sweater and then bring that balloon close to the other balloon, which has not been charged. try this with at least one other object—and for variety in the discussion, avoid using an object already described by your classmates. then, for your initial post to the discussion, answer the following questions: what happened with the two balloons?
Answers: 3
You know the right answer?
The oxidation number of all metals on the periodic table have a positive (+) charge. which of these...
Questions

Biology, 12.10.2019 17:50





Biology, 12.10.2019 17:50


History, 12.10.2019 17:50

English, 12.10.2019 17:50



Chemistry, 12.10.2019 17:50


Mathematics, 12.10.2019 17:50


Health, 12.10.2019 17:50

Mathematics, 12.10.2019 17:50



Mathematics, 12.10.2019 17:50