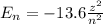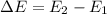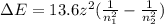How does quantum theory explain the emission spectra of atoms? a. electrons absorb the energy they need to jump to a higher energy level from any light source. b. a single, specific amount of energy is associated with each movement of an electron between energy levels. c. electrons gain and release energy in a slow, continuous fashion. d. the movement of an electron between energy levels occurs gradually as small amounts of energy are absorbed.

Answers: 1
Another question on Physics

Physics, 22.06.2019 17:00
Explain what will happen if decomposers were apsent from a forest ecoysystem.
Answers: 1


Physics, 22.06.2019 19:30
The ability to make things happen is also called a. heat b. force c. matter d. energy
Answers: 1

Physics, 22.06.2019 21:40
Ahair dryer is basically a duct in which a few layers of electric resistors are placed. a small fan pulls the air in and forces it through the resistors where it is heated. air enters a 1200 w hair dryer at 100 kpa and 22°c and leaves at 47°c. the cross-sectional area of the hair dryer at the exit is 60 cm2. neglecting the power consumed by the fan and the heat losses through the walls of the hair dryer, determine (a) the volume flow rate of air at the inlet and (b) the velocity of the air at the exit.
Answers: 1
You know the right answer?
How does quantum theory explain the emission spectra of atoms? a. electrons absorb the energy they...
Questions

Mathematics, 29.08.2021 22:50

Mathematics, 29.08.2021 22:50


Chemistry, 29.08.2021 22:50

Mathematics, 29.08.2021 22:50

History, 29.08.2021 22:50






Chemistry, 29.08.2021 22:50


Mathematics, 29.08.2021 22:50

Mathematics, 29.08.2021 22:50

Mathematics, 29.08.2021 22:50

Mathematics, 29.08.2021 22:50

Mathematics, 29.08.2021 22:50


English, 29.08.2021 22:50