
Physics, 22.07.2019 08:30 Karamatullah
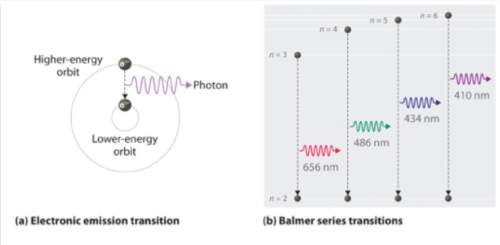
As mentioned in this week’s notes on page 4, the electrons of an atom can occupy different energy shells within the atom (similar to how the planets all occupy different orbits around the sun). electrons prefer to be in the lowest energy shell possible (the ground state); however, they can gain energy and jump to a higher shell by absorbing light or being excited by an electric current. in accordance with the conservation of energy, if an electron drops from a higher energy level to a lower one, this must emit a photon (particle of light) with energy equal to the energy difference of the shells. a balmer series transition is any transition of an electron from some higher energy shell down to the second lowest energy shell (n=2) in hydrogen. looking at image (b) above, what is the wavelength of a photon emitted during the balmer transition from the n=3 shell in hydrogen? (remember nm is short for a nanometer, for example 656 nm = 656 x 10-9 meters) a) 656e-9 meters b) 486e-9 meters c) 434e-9 meters d) 410e-9 meters

Answers: 1
Another question on Physics

Physics, 21.06.2019 21:50
Apossible explanation for a set of facts that can be tested by further investigation is called
Answers: 1


Physics, 22.06.2019 18:00
Which is the most accurate name for the ionic compound cas?
Answers: 1

You know the right answer?
As mentioned in this week’s notes on page 4, the electrons of an atom can occupy different energy sh...
Questions

Health, 28.01.2020 15:00



History, 28.01.2020 15:00

Social Studies, 28.01.2020 15:00

Mathematics, 28.01.2020 15:00

History, 28.01.2020 15:00


World Languages, 28.01.2020 15:00


Biology, 28.01.2020 15:00


Computers and Technology, 28.01.2020 15:00

History, 28.01.2020 15:01


Mathematics, 28.01.2020 15:01



Health, 28.01.2020 15:01

History, 28.01.2020 15:01



