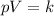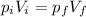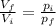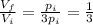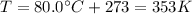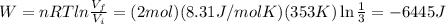Physics, 21.07.2019 11:30 davestrider404
Two moles of an ideal gas are compressed in a cylinder at a constant temperature of 80.0 ∘c until the original pressure has tripled. calculate the amount of work done by gas.

Answers: 1
Another question on Physics

Physics, 21.06.2019 13:00
Alexander calder's mobiles are an example of motion because it uses forces of wind and water.
Answers: 2

Physics, 22.06.2019 16:00
What is the freezing point of radiator fluid that is 50% antifreeze by mass? kf for water is 1.86 ∘c/m.
Answers: 3

Physics, 22.06.2019 17:50
Which of the following best describes internal energy? a. the difference between the kinetic and potential energies of the particles in a system b. the sum of the kinetic and potential energies of the particles in a system c. the sum of the kinetic and thermal energies of the particles in a system d. the difference between the kinetic and thermal energies of the particles in a system
Answers: 2

Physics, 23.06.2019 01:30
The two most prominent wavelengths in the light emitted by a hydrogen discharge lamp are 656(red) and 486 (blue). light from a hydrogen lamp illuminates a diffraction grating with 500 lines per mm, and the light is observed on a screen 1.50 behind the grating. what is the distance between the first-order red and blue fringes?
Answers: 3
You know the right answer?
Two moles of an ideal gas are compressed in a cylinder at a constant temperature of 80.0 ∘c until th...
Questions



Mathematics, 16.04.2020 22:27

Mathematics, 16.04.2020 22:27

Mathematics, 16.04.2020 22:27

Mathematics, 16.04.2020 22:27




History, 16.04.2020 22:27


Mathematics, 16.04.2020 22:28


Mathematics, 16.04.2020 22:28




Medicine, 16.04.2020 22:28


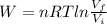 (1)
(1)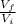 is the ratio between the final volume and the initial volume of the gas
is the ratio between the final volume and the initial volume of the gas