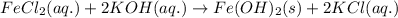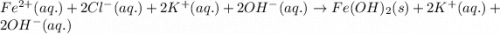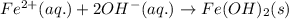Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 09:00
The concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point
Answers: 3
You know the right answer?
Write and balance a net ionic equation for the reaction between iron(ii) chloride and potassium hydr...
Questions

Computers and Technology, 27.09.2019 19:30

Computers and Technology, 27.09.2019 19:30

Computers and Technology, 27.09.2019 19:30

Computers and Technology, 27.09.2019 19:30


Mathematics, 27.09.2019 19:30

Mathematics, 27.09.2019 19:30





Mathematics, 27.09.2019 19:30






Business, 27.09.2019 19:30

Physics, 27.09.2019 19:30