
Chemistry, 19.07.2019 13:00 alejandra216
For the reaction: h2(g) + cl2(g) → 2hcl(g), how many moles hcl will be produced from 10.0 g of h2? the reaction occurs in the presence of excess cl2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
For the reaction: h2(g) + cl2(g) → 2hcl(g), how many moles hcl will be produced from 10.0 g of h2?...
Questions

Mathematics, 01.06.2021 18:40


Social Studies, 01.06.2021 18:40


Biology, 01.06.2021 18:40

Geography, 01.06.2021 18:40


Biology, 01.06.2021 18:40

English, 01.06.2021 18:40

English, 01.06.2021 18:40





Health, 01.06.2021 18:40




History, 01.06.2021 18:40

Mathematics, 01.06.2021 18:40

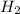 = 10 g
= 10 g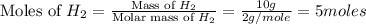
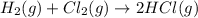
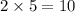 moles of HCl
moles of HCl

