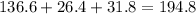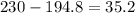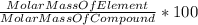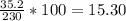Chemistry, 17.07.2019 07:30 sarahbennett11p4yxlb
A230 g sample of a compound contains 136.6 g of carbon, 26.4 g of hydrogen, and 31.8 g of nitrogen. the rest is oxygen. what is the mass percent of oxygen in the compound? 11.48%

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1


Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
A230 g sample of a compound contains 136.6 g of carbon, 26.4 g of hydrogen, and 31.8 g of nitrogen....
Questions






Social Studies, 16.01.2020 05:31

Social Studies, 16.01.2020 05:31


Social Studies, 16.01.2020 05:31











English, 16.01.2020 05:31