
Chemistry, 16.07.2019 04:00 cmfield8810
Hno3, a strong acid, is added to shift the ag2co3 equilibrium (equation 7.6) to the right. explain why the shift occurs. b. what would have been observed if hcl (also a strong acid) had been added instead of the hno3?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Hno3, a strong acid, is added to shift the ag2co3 equilibrium (equation 7.6) to the right. explain w...
Questions

Biology, 25.09.2019 23:00



English, 25.09.2019 23:10



English, 25.09.2019 23:10

History, 25.09.2019 23:10

Computers and Technology, 25.09.2019 23:10

Spanish, 25.09.2019 23:10

Mathematics, 25.09.2019 23:10

Mathematics, 25.09.2019 23:10

Spanish, 25.09.2019 23:10




Social Studies, 25.09.2019 23:10

Social Studies, 25.09.2019 23:10

Social Studies, 25.09.2019 23:10

Social Studies, 25.09.2019 23:10

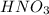 reacts with
reacts with 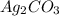 usually the equilibrium shifts to the right because
usually the equilibrium shifts to the right because 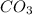 molecule from the reaction mixture, which then tends to shift the equilibrium towards right.
molecule from the reaction mixture, which then tends to shift the equilibrium towards right.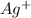 and
and 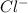 ions from the solution.
ions from the solution.

