
Ethyne (c2 h2 (g), hf = 226.77 kj/mol) undergoes complete combustion in the presence of oxygen to produce carbon dioxide (co2 (g), hf = –393.5 kj/mol ) and water (h2 o(g), hf = –241.82 kj/mol) according to the equation below.what is the enthalpy of combustion (per mole) of c2 h2 (g)? use 

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
Ethyne (c2 h2 (g), hf = 226.77 kj/mol) undergoes complete combustion in the...
Questions

Mathematics, 17.03.2020 22:05




History, 17.03.2020 22:05


Spanish, 17.03.2020 22:05


Biology, 17.03.2020 22:05




Business, 17.03.2020 22:05



Mathematics, 17.03.2020 22:05


Mathematics, 17.03.2020 22:05


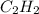 is -1255.6 KJ/mol
is -1255.6 KJ/mol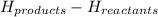

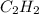 which is -2511.2 KJ/mol
which is -2511.2 KJ/mol

