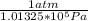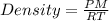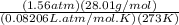Chemistry, 14.07.2019 16:30 Poohlobster
Carbon monoxide is a gas at 0 °c and a pressure of 1.58 × 105 pa. it is a diatomic gas, each of its molecules consisting of one carbon atom (atomic mass = 12.0 u) and one oxygen atom (atomic mass = 16.0 u). assuming that carbon monoxide is an ideal gas, calculate its density ρ.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Carbon monoxide is a gas at 0 °c and a pressure of 1.58 × 105 pa. it is a diatomic gas, each of its...
Questions

History, 10.07.2019 07:40

Biology, 10.07.2019 07:40

History, 10.07.2019 07:40


History, 10.07.2019 07:40




Mathematics, 10.07.2019 07:40


History, 10.07.2019 07:40





Mathematics, 10.07.2019 07:40

Health, 10.07.2019 07:40



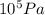 ×
×