Chemistry, 14.07.2019 15:00 Nickanderson21
According to the first law of thermodynamics, the amount of work done by a heat engine equals the amount of a. work done on the engine. b. waste heat it produces. c. thermal energy added to the engine minus the waste heat. d. thermal energy added to the engine plus the waste heat.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3


Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
According to the first law of thermodynamics, the amount of work done by a heat engine equals the am...
Questions

Physics, 21.12.2020 14:00

English, 21.12.2020 14:00


English, 21.12.2020 14:00

Biology, 21.12.2020 14:00


English, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00




English, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00

Chemistry, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00


Mathematics, 21.12.2020 14:00



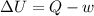
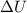 = Internal energy
= Internal energy