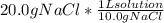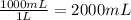Chemistry, 14.07.2019 10:00 isabelleecurtis
Astudent is told to use 20.0 grams of sodium chloride to make an aqueous solution that has a concentration of 10.0 grams of sodium chloride per liter of solution. assuming that 20.0 grams of sodium chloride has a volume of 7.5 milliliters, about how much water will she use in making this solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
Astudent is told to use 20.0 grams of sodium chloride to make an aqueous solution that has a concent...
Questions


Mathematics, 05.05.2020 17:37



Computers and Technology, 05.05.2020 17:37

Physics, 05.05.2020 17:37






English, 05.05.2020 17:37







Health, 05.05.2020 17:37

Computers and Technology, 05.05.2020 17:37