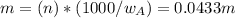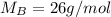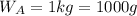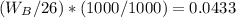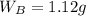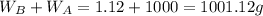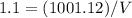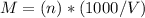Chemistry, 11.07.2019 19:30 isanaty7951
Asolution is 0.0433 m lif. what is the molarity of the solution if the density is 1.10 g/ml

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Asolution is 0.0433 m lif. what is the molarity of the solution if the density is 1.10 g/ml...
Questions

English, 07.03.2020 22:52

History, 07.03.2020 22:53






Social Studies, 07.03.2020 22:54

History, 07.03.2020 22:54

Mathematics, 07.03.2020 22:55



English, 07.03.2020 22:55



Mathematics, 07.03.2020 22:57


History, 07.03.2020 22:57

Mathematics, 07.03.2020 22:57

Mathematics, 07.03.2020 22:58