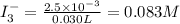Chemistry, 11.07.2019 19:00 imalexiscv
What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is required to completely react with 25.00 ml of a 0.200 m thiosulfate solution, k2s2o3(aq)? the chemical equation for the reaction is 2 s2o32-(aq) + i3-(aq) → s4o62-(aq) + 3 i-(aq). what is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is required to completely react with 25.00 ml of a 0.200 m thiosulfate solution, k2s2o3(aq)? the chemical equation for the reaction is 2 s2o32-(aq) + i3-(aq) → s4o62-(aq) + 3 i-(aq). 0.167 m 0.333 m 0.120 m 0.0833 m?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 08:00
The biosphere of the earth is made up of . a. inorganic b. organic
Answers: 2
You know the right answer?
What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is requ...
Questions


English, 20.08.2019 07:10




English, 20.08.2019 07:10

Health, 20.08.2019 07:10



Mathematics, 20.08.2019 07:10




History, 20.08.2019 07:10

Mathematics, 20.08.2019 07:10

Biology, 20.08.2019 07:10

Advanced Placement (AP), 20.08.2019 07:10


Mathematics, 20.08.2019 07:10

Mathematics, 20.08.2019 07:10

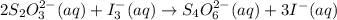
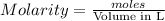
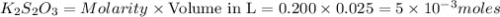
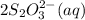 require 1 mole of
require 1 mole of 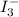
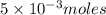 require=
require=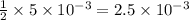 moles of
moles of