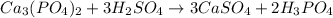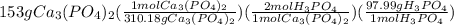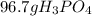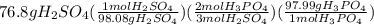Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
Suppose the reaction ca3(po4)2 + 3h2so4 ® 3caso4 + 2h3po4 is carried out starting with 153 g of ca3(...
Questions

Computers and Technology, 26.07.2019 05:50

Computers and Technology, 26.07.2019 05:50


Business, 26.07.2019 05:50

Social Studies, 26.07.2019 05:50

Social Studies, 26.07.2019 05:50


Biology, 26.07.2019 05:50

Mathematics, 26.07.2019 05:50

History, 26.07.2019 05:50

Biology, 26.07.2019 05:50


History, 26.07.2019 05:50


History, 26.07.2019 05:50

Biology, 26.07.2019 05:50

Mathematics, 26.07.2019 05:50


Mathematics, 26.07.2019 05:50

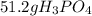 .
.