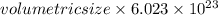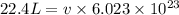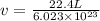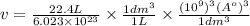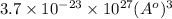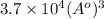Calculate the volumetric size of a water molecule in water vapor at normal conditions, assuming 1 mole of the vapor occupies 22.4 l, as if the vapor were an ideal gas. give answer in angstroms, two significant digits. do not write down units in your answer. calculate the volumetric size of a water molecule in water vapor at normal conditions, assuming 1 mole of the vapor occupies 22.4 l, as if the vapor were an ideal gas. give answer in angstroms, two significant digits. do not write down units in your answer.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1


Chemistry, 23.06.2019 21:50
Arunner wants to run 13.1 km . she knows that her running pace is 6.2 mi/h .how many minutes must she run? hint: use 6.2 mi/h as a conversion factor between distance and time.
Answers: 2
You know the right answer?
Calculate the volumetric size of a water molecule in water vapor at normal conditions, assuming 1 mo...
Questions





History, 14.03.2020 20:12



Law, 14.03.2020 20:13


Mathematics, 14.03.2020 20:13

Mathematics, 14.03.2020 20:13

Mathematics, 14.03.2020 20:13




Mathematics, 14.03.2020 20:14



English, 14.03.2020 20:15

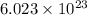 molecules of water.
molecules of water.