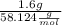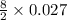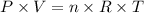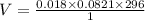Butane, c4h10, is a component of natural gas that is used as fuel for cigarette lighters. the balanced equation of the complete combustion of butane is 2c4h10(g)+13o2(g)→8co2(g)+10h2o(l) at 1.00 atm and 23 ∘c, what is the volume of carbon dioxide formed by the combustion of 1.60 g of butane?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
You know the right answer?
Butane, c4h10, is a component of natural gas that is used as fuel for cigarette lighters. the balanc...
Questions


Mathematics, 29.07.2019 20:00

Mathematics, 29.07.2019 20:00


Business, 29.07.2019 20:00

Mathematics, 29.07.2019 20:00

Biology, 29.07.2019 20:00




Mathematics, 29.07.2019 20:00


Biology, 29.07.2019 20:00


Mathematics, 29.07.2019 20:00

Mathematics, 29.07.2019 20:00


Mathematics, 29.07.2019 20:00