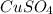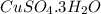Chemistry, 10.07.2019 21:00 saltedcaramel60
In another experiment, you have 4.5000 g of a copper(ii) sulfate hydrate with an unknown number of attached water molecules. after heating the hydrate, you have 3.3608 g of the anhydrous compound (copper(ii) sulfate with no waters) left. using these data, calculate the number of water molecules that is present in the formula of this hydrate (obviously before heating).

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
In another experiment, you have 4.5000 g of a copper(ii) sulfate hydrate with an unknown number of a...
Questions


Biology, 26.08.2020 19:01



Chemistry, 26.08.2020 19:01

History, 26.08.2020 19:01

Geography, 26.08.2020 19:01



Mathematics, 26.08.2020 19:01




English, 26.08.2020 19:01

Physics, 26.08.2020 19:01





Mathematics, 26.08.2020 19:01

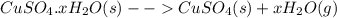
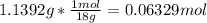
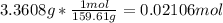
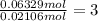
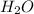 per one mol
per one mol