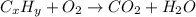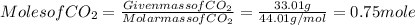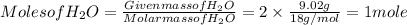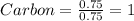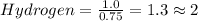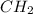Chemistry, 10.07.2019 16:30 hernan99961
Calculate the empirical formula of the hydrocarbon. combustion analysis of a hydrocarbon produced 33.01 g co2 and 9.02 g h2o.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3
You know the right answer?
Calculate the empirical formula of the hydrocarbon. combustion analysis of a hydrocarbon produced 33...
Questions






Mathematics, 21.05.2021 17:30



Mathematics, 21.05.2021 17:30


Mathematics, 21.05.2021 17:30

Mathematics, 21.05.2021 17:30