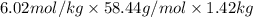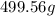Chemistry, 10.07.2019 13:00 iamsecond235p318rq
What mass of salt (nacl) should you add to 1.42 l of water in an ice cream maker to make a solution that freezes at -11.2 ∘c ? assume complete dissociation of the nacl and density of 1.00 g/ml for water?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:30
Which is the most likely way an automotive engineer would use chemistry
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2

Chemistry, 23.06.2019 14:00
Cassandra made a venn diagram to compare and contrast the two stages of cellular respiration. which belongs in the area marked x? energy is released. oxygen is used up. glucose is broken down. carbon dioxide is used up.
Answers: 1
You know the right answer?
What mass of salt (nacl) should you add to 1.42 l of water in an ice cream maker to make a solution...
Questions









Computers and Technology, 27.11.2019 00:31




Computers and Technology, 27.11.2019 00:31







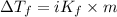
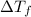 = depression in freezing point
= depression in freezing point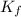 = molal depression constant (
= molal depression constant (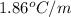 )
)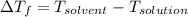
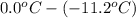
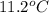
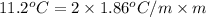 (as sodium chloride dissociate into two ions, i =2)
(as sodium chloride dissociate into two ions, i =2)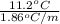
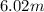
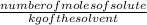
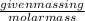
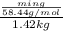 (1 L = 1kg)
(1 L = 1kg)