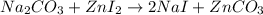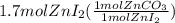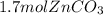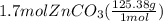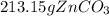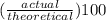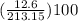Chemistry, 10.07.2019 07:30 takaralocklear
Areaction between 1.7 moles of zinc iodide and excess sodium carbonate yields 12.6 grams of zinc carbonate. this is the equation for the reaction: na2co3 + zni2 → 2nai + znco3. what is the percent yield of zinc carbonate? the percent yield of zinc carbonate is %.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
Areaction between 1.7 moles of zinc iodide and excess sodium carbonate yields 12.6 grams of zinc car...
Questions

Spanish, 30.03.2020 19:04






Computers and Technology, 30.03.2020 19:04




Health, 30.03.2020 19:04

Mathematics, 30.03.2020 19:04





English, 30.03.2020 19:04



Computers and Technology, 30.03.2020 19:05