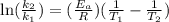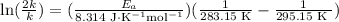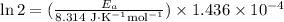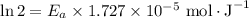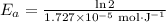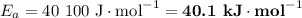Chemistry, 07.07.2019 22:00 gonzalesalexiaouv1bg
If a temperature increase from 10.0 ∘c to 22.0 ∘c doubles the rate constant for a reaction, what is the value of the activation barrier for the reaction? \

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
If a temperature increase from 10.0 ∘c to 22.0 ∘c doubles the rate constant for a reaction, what is...
Questions




Mathematics, 16.03.2020 21:21


Computers and Technology, 16.03.2020 21:21

Chemistry, 16.03.2020 21:21


Mathematics, 16.03.2020 21:21

Mathematics, 16.03.2020 21:21




Mathematics, 16.03.2020 21:21



Health, 16.03.2020 21:21

Mathematics, 16.03.2020 21:21

Mathematics, 16.03.2020 21:21