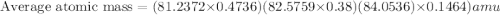Chemistry, 05.07.2019 08:30 aduncan3426
The element loncapium has three isotopes. the first lc-82 has a mass of 81.2372 amu and makes up 47.36% of a standard sample. the second isotope, lc-83, has a mass of 82.5759 amu and makes up 38% of a standard sample. the third isotope, lc-85 has a mass of 84.0536 amu and makes up the remainder of the sample. what is the average atomic mass of this element?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
You know the right answer?
The element loncapium has three isotopes. the first lc-82 has a mass of 81.2372 amu and makes up 47....
Questions

English, 29.01.2021 22:30




Mathematics, 29.01.2021 22:30

Mathematics, 29.01.2021 22:30



Chemistry, 29.01.2021 22:30

Advanced Placement (AP), 29.01.2021 22:30

English, 29.01.2021 22:30

Advanced Placement (AP), 29.01.2021 22:30



Mathematics, 29.01.2021 22:30


History, 29.01.2021 22:30



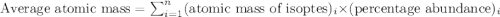 ....(1)
....(1)