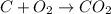Chemistry, 04.07.2019 09:30 smithmalyk4
View the diagram below describe what happened in the diagram. the molecules on both sides of the chemical reaction are the same, because they are both mixtures. bonds were broken on the reactants and new bonds were formed on the products. the molecules expanded and cooled. bonds were broken on the reactants and no new bonds were formed on the products.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1
You know the right answer?
View the diagram below describe what happened in the diagram. the molecules on both sides of the che...
Questions

Business, 05.11.2020 17:50



English, 05.11.2020 17:50



Chemistry, 05.11.2020 17:50

Social Studies, 05.11.2020 17:50

Mathematics, 05.11.2020 17:50



Health, 05.11.2020 17:50

Mathematics, 05.11.2020 17:50

Arts, 05.11.2020 17:50

Mathematics, 05.11.2020 17:50

English, 05.11.2020 17:50

Social Studies, 05.11.2020 17:50


Mathematics, 05.11.2020 17:50

Mathematics, 05.11.2020 17:50