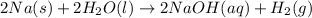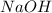Chemistry, 04.07.2019 07:00 Robinlynn228
Assign an oxidation number to each atom in the products. 2na(s)+2h2o(l)→2naoh(aq)+h2(g) assign an oxidization number for: na , o , h (in naoh) , h (in h2) i'm not sure how to assign oxidization numbers, if someone could explain it to me that'd be great! i have an exam that involves oxidization numbers coming up, and i'm completely clueless on them! you in advance!

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1


Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3
You know the right answer?
Assign an oxidation number to each atom in the products. 2na(s)+2h2o(l)→2naoh(aq)+h2(g) assign an ox...
Questions




Mathematics, 24.06.2020 04:01

Mathematics, 24.06.2020 04:01















 is zero.
is zero.