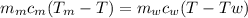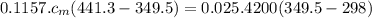Ahot lump of 115.7 g of an unknown substance initially at 168.3°c is placed in 25.0 ml of water initially at 25.0°c and allowed to reach thermal equilibrium. the final temperature of the system is 76.5°c. what is the identity of the unknown substance? assume no heat is lost to the surroundings

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1


Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Ahot lump of 115.7 g of an unknown substance initially at 168.3°c is placed in 25.0 ml of water init...
Questions


English, 13.03.2020 18:06

Mathematics, 13.03.2020 18:06




Geography, 13.03.2020 18:07





Computers and Technology, 13.03.2020 18:07



Computers and Technology, 13.03.2020 18:07


Mathematics, 13.03.2020 18:07

Mathematics, 13.03.2020 18:07


 .
.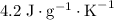 and a density of
and a density of 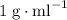 . 25.0 milliliters of water thus has a mass of 25.0 grams.
. 25.0 milliliters of water thus has a mass of 25.0 grams. 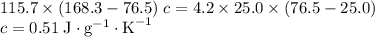
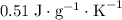 . This substance is thus probably steel.
. This substance is thus probably steel.