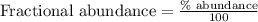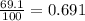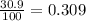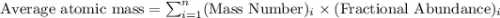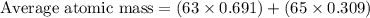Chemistry, 01.07.2019 17:30 eeeeee9848
In a mass spectrum, there are two peaks, one at m/z 63.0 with percentage abundance 69.1%, and another peak at m/z 65.0 with percentage abundance 30.9%. determine the relative atomic mass (ar) for this element, giving the answer to 3 significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
In a mass spectrum, there are two peaks, one at m/z 63.0 with percentage abundance 69.1%, and anothe...
Questions



Mathematics, 01.09.2020 19:01



English, 01.09.2020 19:01




Business, 01.09.2020 19:01


Mathematics, 01.09.2020 19:01



History, 01.09.2020 19:01





Spanish, 01.09.2020 19:01