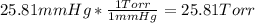Asample of oxygen gas was collected via water displacement. since the oxygen was collected via water displacement, the sample is saturated with water vapor. if the total pressure of the mixture at 26.4 °c is 749 torr, what is the partial pressure of oxygen? the vapor pressure of water at 26.4 °c is 25.81 mm hg.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 09:00
What sources of error may have contributed to the percent yield not being 100 percent? think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.
Answers: 2
You know the right answer?
Asample of oxygen gas was collected via water displacement. since the oxygen was collected via water...
Questions

Social Studies, 03.03.2021 01:30




Mathematics, 03.03.2021 01:30




Mathematics, 03.03.2021 01:30


History, 03.03.2021 01:30

Biology, 03.03.2021 01:30



Mathematics, 03.03.2021 01:30




Chemistry, 03.03.2021 01:30

History, 03.03.2021 01:30

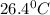 =25.81mmHg
=25.81mmHg