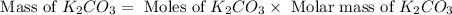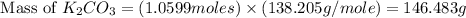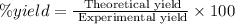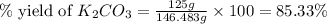Chemistry, 29.06.2019 21:30 vannaht2003
Given the following reaction: 2k3po4 + al2(co3)3 = 3k2co3 + 2alpo4 if i perform this reaction with 150 g of potassium phosphate and 90 g of aluminum carbonate, what is my theoretical yeild in grams of potassium carbonate? if the reaction results in 125 g of potassium carbonate, what is my percent yeild?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:40
Enough of a monoprotic weak acid is dissolved in water to produce a 0.0172 m solution. if the ph of the resulting solution is 2.39 at 20 °c, determine the pka for the acid.
Answers: 1

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Given the following reaction: 2k3po4 + al2(co3)3 = 3k2co3 + 2alpo4 if i perform this reaction with...
Questions

Mathematics, 07.11.2019 06:31



Health, 07.11.2019 06:31

Mathematics, 07.11.2019 06:31



Mathematics, 07.11.2019 06:31

History, 07.11.2019 06:31





Health, 07.11.2019 06:31






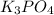 = 150 g
= 150 g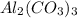 = 90 g
= 90 g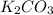 = 138.205 g/mole
= 138.205 g/mole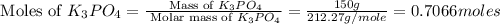
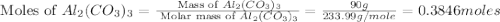
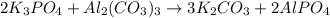
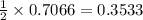 moles of
moles of 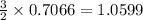 moles of
moles of