
Chemistry, 29.06.2019 17:00 officialalex6330
Both stearic acid and sucrose are molecular solids, but one is polar and the other is non polar. compare the solubility of the two compounds in water and in hexane to determine which is which

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2


Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Both stearic acid and sucrose are molecular solids, but one is polar and the other is non polar. com...
Questions

Mathematics, 26.05.2021 19:20


Mathematics, 26.05.2021 19:20


English, 26.05.2021 19:20

Mathematics, 26.05.2021 19:20



Mathematics, 26.05.2021 19:20


Health, 26.05.2021 19:20





Mathematics, 26.05.2021 19:20

Mathematics, 26.05.2021 19:20



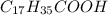 . The hydrophobic part is this chain is made up of 17 carbon atom and hydrophilic part is only of made up of carboxylic group due to which it won't get dissolve in water.Which means that stearic acid will get dissolve in non polar solvent that is hexane. Hence, stearic acid is non polar molecule.
. The hydrophobic part is this chain is made up of 17 carbon atom and hydrophilic part is only of made up of carboxylic group due to which it won't get dissolve in water.Which means that stearic acid will get dissolve in non polar solvent that is hexane. Hence, stearic acid is non polar molecule.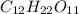 .Due to presence of polar hydroxyl(-OH) groups in molecule , molecule will get easily dissolve in polar solvent that is water. Since,the hydrophobic part in the molecules are covered by the hydrophilic part due to which it gets easily dissolve in water. Hence, the sucrose in polar molecule.
.Due to presence of polar hydroxyl(-OH) groups in molecule , molecule will get easily dissolve in polar solvent that is water. Since,the hydrophobic part in the molecules are covered by the hydrophilic part due to which it gets easily dissolve in water. Hence, the sucrose in polar molecule.

