
Chemistry, 29.06.2019 12:30 yairreyes01
Which of the following statements describes the results of this reaction? c6h12o6 + 6 o2 → 6 co2 + 6 h2o + energy a) c6h12o6 is oxidized and o2 is reduced. b) o2 is oxidized and h2o is reduced. c) co2 is reduced and o2 is oxidized. d) c6h12o6is reduced and co2 is oxidized. e) o2 is reduced and co2 is oxidized.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2


Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
Which of the following statements describes the results of this reaction? c6h12o6 + 6 o2 → 6 co2 +...
Questions






Social Studies, 11.10.2019 06:20

Biology, 11.10.2019 06:20

Mathematics, 11.10.2019 06:20

Chemistry, 11.10.2019 06:20

Mathematics, 11.10.2019 06:20




Physics, 11.10.2019 06:20




Mathematics, 11.10.2019 06:20


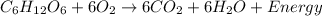
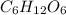 is getting oxidized.
is getting oxidized.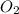 is getting reduced.
is getting reduced.


