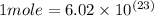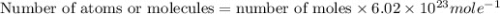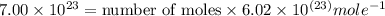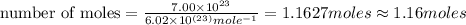Chemistry, 28.06.2019 20:30 truthqmatic16
In the world of chemistry, a 'mole' is a number of a very large number of something. just as a 'dozen' is 12 of something, a 'mole' is about 6.02 * 10^23 of something. in chemistry, we like to measure the number of atoms or molecules in moles. if one mole is equal to 6.02 * 10^23 atoms, and you have 7.00 * 10^23 atoms, then how many moles do you have? round your answer to two decimal places.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3


Chemistry, 23.06.2019 09:00
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2
You know the right answer?
In the world of chemistry, a 'mole' is a number of a very large number of something. just as a 'do...
Questions

Mathematics, 20.04.2020 19:35

Social Studies, 20.04.2020 19:35

Spanish, 20.04.2020 19:35

Mathematics, 20.04.2020 19:35

Mathematics, 20.04.2020 19:35






Mathematics, 20.04.2020 19:36

History, 20.04.2020 19:36



English, 20.04.2020 19:36

Mathematics, 20.04.2020 19:36

Mathematics, 20.04.2020 19:36



 numbers of atoms.
numbers of atoms.