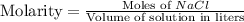Chemistry, 28.06.2019 20:00 carlosiscr7
What is true of a solution of 2.0 m nacl(aq)? it contains 2.0 grams of nacl per 100 grams of water. it contains 2.0 grams of nacl per 1 liter of solution. it contains 2.0 moles of nacl per 100 grams of water. it contains 2.0 moles of nacl per 1 liter of solution.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:10
Cobalt-60 is an artificial radioisotope that is produced in a nuclear reactor and is used as a gamma-ray source in the treatment of certain types of cancer. if the wavelength of the gamma radiation from a cobalt-60 source is 1.00 × 10-3 nm, calculate the energy of a photon of this radiation.
Answers: 2

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1
You know the right answer?
What is true of a solution of 2.0 m nacl(aq)? it contains 2.0 grams of nacl per 100 grams of water....
Questions


Computers and Technology, 07.10.2019 03:30


Mathematics, 07.10.2019 03:30

History, 07.10.2019 03:30

Mathematics, 07.10.2019 03:30


Mathematics, 07.10.2019 03:30

Business, 07.10.2019 03:30

Biology, 07.10.2019 03:30

Arts, 07.10.2019 03:30

Mathematics, 07.10.2019 03:30



Mathematics, 07.10.2019 03:30


History, 07.10.2019 03:30

Biology, 07.10.2019 03:30


Mathematics, 07.10.2019 03:30