
Chemistry, 07.10.2019 22:30 grantjaylynn
At very high temperatures, copper(ii) sulfate undergoes the reaction cuso4(s) --> cuo (s) + so3 (g)
what kind of reaction is this?
if 319.22 g cuso4(s) reacted completely in the reaction, how many grams of cuo(s) would be produced?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1


Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
At very high temperatures, copper(ii) sulfate undergoes the reaction cuso4(s) --> cuo (s) + so3...
Questions

English, 24.02.2021 21:40

History, 24.02.2021 21:40


English, 24.02.2021 21:40

Mathematics, 24.02.2021 21:40


Mathematics, 24.02.2021 21:40


Mathematics, 24.02.2021 21:40

Mathematics, 24.02.2021 21:40



Mathematics, 24.02.2021 21:40


Computers and Technology, 24.02.2021 21:40


Mathematics, 24.02.2021 21:40

Mathematics, 24.02.2021 21:40

Mathematics, 24.02.2021 21:40

History, 24.02.2021 21:40


 gives 1 mole of
gives 1 mole of 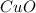 .
. 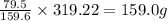 of
of 

