Chemistry, 29.01.2020 17:45 firdausmohammed80
Reviewing for a test - need an answer and explanation.
base your answer to the following question on the information below.
the reaction between aluminum and an aqueous solution of gold (i) sulfate is represented by the unbalanced equation below.
al (s) + au2so4 (aq) -> al2(so4)3 (aq) + au (s)
determine the total mass of au sproduced when 6.52 grams of al reacts completely with 3.58 grams of au2so4 to produce 5.95 grams of al2(so4)3.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:10
The rock in a lead ore deposit contains 89 % pbs by mass. how many kilograms of the rock must be processed to obtain 1.5 kg of pb?
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
Reviewing for a test - need an answer and explanation.
base your answer to the following...
base your answer to the following...
Questions

Biology, 28.06.2019 08:30



Mathematics, 28.06.2019 08:30


Chemistry, 28.06.2019 08:30



Computers and Technology, 28.06.2019 08:30

Mathematics, 28.06.2019 08:30

Computers and Technology, 28.06.2019 08:30



Mathematics, 28.06.2019 08:30





Chemistry, 28.06.2019 08:30

Social Studies, 28.06.2019 08:30

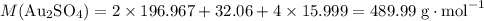 .
.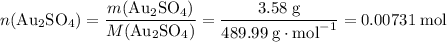 .
.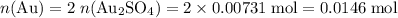 .
.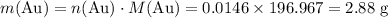 .
.