
Chemistry, 15.10.2019 19:30 Gimagg5549
The iodine "clock reaction" involves the following sequence of reactions occurring in a reaction mixture in a single beaker. 1. io3(aq) + 5i–(aq) + 6h+(aq) → 3i2(aq) + 3h2o(l) 2. i2(aq) + 2s2o32–(aq) → 2i–(aq) + s4o62–(aq) the molecular iodine (i2) formed in reaction 1 is immediately used up in reaction 2, so that no iodine accumulates. in one experiment, a student made up a reaction mixture which initially contained 0.0020 mol of iodate ions (io3–). if the iodate ions reacted completely, how many moles of thiosulfate ions (s2o32–) were needed in reaction 2, in order to react completely with the iodine (i2) produced in reaction 1? multiple choice 0.0020 mol 0.012 mol 0.0040 mol

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:50
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

You know the right answer?
The iodine "clock reaction" involves the following sequence of reactions occurring in a reaction mix...
Questions


Physics, 06.01.2020 19:31






Mathematics, 06.01.2020 19:31

Computers and Technology, 06.01.2020 19:31

Mathematics, 06.01.2020 19:31



Engineering, 06.01.2020 19:31







Mathematics, 06.01.2020 19:31

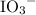 .
.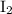 will be produced?
will be produced?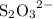 are required?
are required?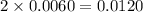 moles of
moles of 

