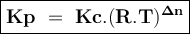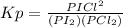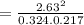Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

You know the right answer?
Consider the reaction: i2(g)+cl2(g)⇌2icl(g) kp= 81.9 at 25 ∘c. calculate δgrxn for the reaction at...
Questions













Computers and Technology, 27.11.2019 02:31







![\large {\boxed {\bold {Kc ~ = ~ \frac {[C] ^ m [D] ^ n} {[A] ^ p [B] ^ q}}}}](/tpl/images/0425/9888/55d71.png)
![\large {\boxed {\bold {Kp ~ = ~ \frac {[pC] ^ m [pD] ^ n} {[pA] ^ p [pB] ^ q}}}}](/tpl/images/0425/9888/1041d.png)