Chemistry, 04.01.2020 14:31 battlemarshmell
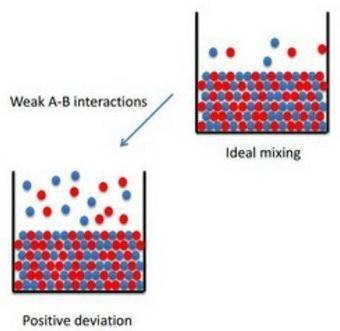
How to apply raoult's law to real solutions consider mixing a liquid with a vapor pressure of 100 torr with an equimolar amount of a liquid with a vapor pressure of 200 torr. the resulting solution would be predicted to have a vapor pressure of 150 torr if it behaved ideally. if, however, the interactions between the different components are not similar we can see positive or negative deviations from the calculated vapor pressure. an actual vapor pressure greater than that predicted by raoult's law is said to be a positive deviation and an actual vapor pressure lower than that predicted by raoult's law is a negative deviation. part a imagine a solution of two liquids in which the molecules interact less favorably than they do in the individual liquids. will this solution deviate positively from, deviate negatively from, or ideally follow raoult's law? '

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
How to apply raoult's law to real solutions consider mixing a liquid with a vapor pressure of 100 to...
Questions

Chemistry, 11.12.2020 01:00


Mathematics, 11.12.2020 01:00




Arts, 11.12.2020 01:00




History, 11.12.2020 01:00




Mathematics, 11.12.2020 01:00



Mathematics, 11.12.2020 01:00