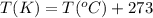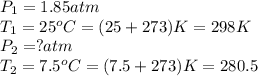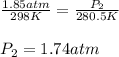Chemistry, 22.06.2019 06:00 giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if th...
Questions


Physics, 20.08.2019 16:10

Mathematics, 20.08.2019 16:10

Mathematics, 20.08.2019 16:10




Mathematics, 20.08.2019 16:10

Computers and Technology, 20.08.2019 16:10


Social Studies, 20.08.2019 16:10




Geography, 20.08.2019 16:10



Health, 20.08.2019 16:10

History, 20.08.2019 16:10

History, 20.08.2019 16:10

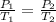 (at constant volume)
(at constant volume)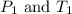 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.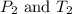 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.