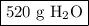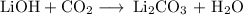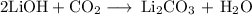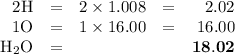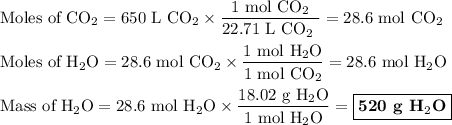Chemistry, 28.06.2019 04:10 lilymessina94
Answer the following question: in a space shuttle, the co2 that the crew exhales is removed from the air by a reaction within canisters of lithium hydroxide. on average, each astronaut exhales about 650 l of co2 daily. what mass of water will be produced
when this amount reacts with lioh? the other product of the reaction is
li2co3. when answering this question include the following:
have both the unbalanced and balanced chemical equations.
explain how to find the molar mass of the compounds.
explain how the balanced chemical equation is used to find the ratio of moles (hint: step 3 in the video).
explain how many significant figures your answer needs to have.
the numerical answer

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
Answer the following question: in a space shuttle, the co2 that the crew exhales is removed from th...
Questions


English, 31.07.2019 05:00




Spanish, 31.07.2019 05:00

Social Studies, 31.07.2019 05:00

Mathematics, 31.07.2019 05:00





History, 31.07.2019 05:00

Health, 31.07.2019 05:00

English, 31.07.2019 05:00

Mathematics, 31.07.2019 05:00

English, 31.07.2019 05:00

Mathematics, 31.07.2019 05:00