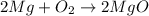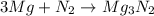Chemistry, 01.07.2019 18:10 jtorres0520
In a common experiment in the general chemistry laboratory, magnesium metal is heated in air to produce mgo. mgo is a white solid, but in these experiments it often looks gray, due to small amounts of mg3n2 , a compound formed as some of the magnesium reacts with nitrogen. write a balanced equation for each reaction.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Figure 10-1 study figure 10-1. the strong nuclear force felt by a single proton in a large nucleus
Answers: 3

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
In a common experiment in the general chemistry laboratory, magnesium metal is heated in air to prod...
Questions



Mathematics, 07.07.2019 22:10

Mathematics, 07.07.2019 22:10




Mathematics, 07.07.2019 22:10




Mathematics, 07.07.2019 22:10

Mathematics, 07.07.2019 22:10

Mathematics, 07.07.2019 22:10

Mathematics, 07.07.2019 22:10





Biology, 07.07.2019 22:10