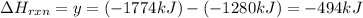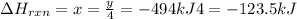Phosphorous pentachloride is used in the industrial preparation of many organic phosphorous compounds. equation i shows its preparation from pcl3 and cl2: (i) pcl3 (l) + cl2(g) pcl5(s) use equation ii and iii to calculate ∆hrxs of equation i: (ii) p4 (s) + 6 cl2 (g) 4 pcl3 (l) ∆h = 1280 kj (iii) p4 (s) + 10 cl2 (g) 4 pcl5 (s) ∆h = 1774 kj

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1


Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
Phosphorous pentachloride is used in the industrial preparation of many organic phosphorous compound...
Questions

Geography, 07.07.2019 11:50



English, 07.07.2019 11:50

Physics, 07.07.2019 11:50


Mathematics, 07.07.2019 11:50

Mathematics, 07.07.2019 11:50

Mathematics, 07.07.2019 11:50




Mathematics, 07.07.2019 11:50

Mathematics, 07.07.2019 11:50

Mathematics, 07.07.2019 11:50

Mathematics, 07.07.2019 11:50

History, 07.07.2019 11:50

Mathematics, 07.07.2019 11:50


Mathematics, 07.07.2019 11:50

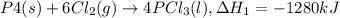 ..(1)
..(1)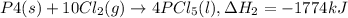 ..(2)
..(2)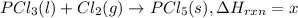 ...(3)
...(3)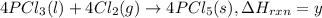
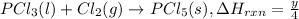 ...(3)
...(3)