
Chemistry, 02.07.2019 01:10 jen12abc82
Which of the following has the greatest electronegativity difference between the bonded atoms? view available hint(s) which of the following has the greatest electronegativity difference between the bonded atoms? a strong acid made of hydrogen and a halogen, such as hcl a group 1 alkali metal bonded to fluoride, such as lif. carbon bonded to a group 6a (16) nonmetal chalcogen, such as in co a diatomic gas, such as nitrogen (n2).

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2


Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Which of the following has the greatest electronegativity difference between the bonded atoms? view...
Questions


Mathematics, 13.06.2021 04:10




History, 13.06.2021 04:10

English, 13.06.2021 04:10



Mathematics, 13.06.2021 04:10


Computers and Technology, 13.06.2021 04:10


Mathematics, 13.06.2021 04:10

English, 13.06.2021 04:10

Mathematics, 13.06.2021 04:10

Mathematics, 13.06.2021 04:10


Arts, 13.06.2021 04:10

Arts, 13.06.2021 04:10

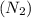 : Non-polar covalent bond is defined as the bond which is formed when there is no difference of electronegativities between the atoms.
: Non-polar covalent bond is defined as the bond which is formed when there is no difference of electronegativities between the atoms.

