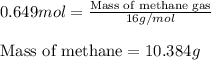Consider the following reaction: 2ch3oh(g) 2ch4(g) + o2(g) δh = +252.8 kj a) calculate the amount of heat transferred when 24.0 g of ch3oh(g) is decomposed by this reaction at constant pressure. b) for a given sample of ch3oh, the enthalpy change during the reaction is 82.1 kj. how many grams of methane gas are produced?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

You know the right answer?
Consider the following reaction: 2ch3oh(g) 2ch4(g) + o2(g) δh = +252.8 kj a) calculate the amount...
Questions



Computers and Technology, 28.12.2019 01:31



Social Studies, 28.12.2019 01:31



Computers and Technology, 28.12.2019 01:31











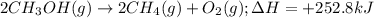
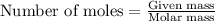 ......(1)
......(1)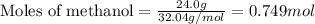
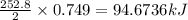
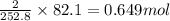 of methane gas is produced.
of methane gas is produced.