Chemistry, 12.07.2019 19:10 deonnaturner68p7hz7y
Glucose, c6h12o6, is used as an energy source by the human body. the overall reaction in the body is described by the equation c6h12o6(aq)+6o2(g)⟶6co2(g)+6h2o(l) calculate the number of grams of oxygen required to convert 48.0 g of glucose to co2 and h2o. mass of o2: g calculate the number of grams of co2 produced. mass of co2: g

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1

You know the right answer?
Glucose, c6h12o6, is used as an energy source by the human body. the overall reaction in the body is...
Questions

Mathematics, 26.09.2021 23:00



English, 26.09.2021 23:00

Mathematics, 26.09.2021 23:00

Social Studies, 26.09.2021 23:00

Biology, 26.09.2021 23:00

Mathematics, 26.09.2021 23:00




Biology, 26.09.2021 23:00

Mathematics, 26.09.2021 23:00

Mathematics, 26.09.2021 23:00

English, 26.09.2021 23:00



Mathematics, 26.09.2021 23:00

Mathematics, 26.09.2021 23:00


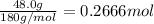
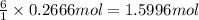 of oxygen
of oxygen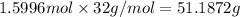
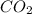 and
and 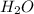 .
.