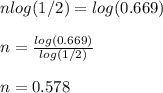Chemistry, 22.07.2019 18:10 sammyraegarrett
The carbon−14 decay rate of a sample obtained from a young tree is 0.266 disintegration per second per gram of the sample. another wood sample prepared from an object recovered at an archaeological excavation gives a decay rate of 0.178 disintegration per second per gram of the sample. what is the age of the object? (the half-life of carbon−14 is 5715 years.) × 10 years enter your answer in scientific notation.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
The carbon−14 decay rate of a sample obtained from a young tree is 0.266 disintegration per second p...
Questions


Chemistry, 17.05.2021 18:50

Mathematics, 17.05.2021 18:50

English, 17.05.2021 18:50

Mathematics, 17.05.2021 18:50


Mathematics, 17.05.2021 18:50

Chemistry, 17.05.2021 18:50




Mathematics, 17.05.2021 18:50

Mathematics, 17.05.2021 18:50




Biology, 17.05.2021 18:50