Chemistry, 29.07.2019 19:20 karatekats1
Use the free energies of formation given below to calculate the equilibrium constant (k) for the following reaction at 298 k. 2 hno3(aq) + no(g) → 3 no2(g) + h2o(l) k = ? δ g0f(kj/mol) -110.9 87.6 51.3 -237.1

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2

Chemistry, 23.06.2019 13:20
What volume of 24% trichloroacetic acid (tca) is needed to prepare eight 3 ounce bottles of 10% tca solution?
Answers: 2
You know the right answer?
Use the free energies of formation given below to calculate the equilibrium constant (k) for the fol...
Questions




History, 03.09.2020 01:01



English, 03.09.2020 01:01

History, 03.09.2020 01:01

Mathematics, 03.09.2020 01:01

Mathematics, 03.09.2020 01:01

Mathematics, 03.09.2020 01:01




Mathematics, 03.09.2020 01:01





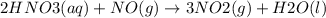
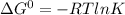
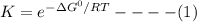
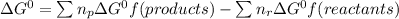
![\Delta G^{0}=[3\Delta G^{0}f(NO2)+3\Delta G^{0}f(H2O)]-[2\Delta G^{0}f(HNO3)+1\Delta G^{0}f(NO)]](/tpl/images/0147/7522/36e5a.png)
![\Delta G^{0}=[3\Delta G^{0}f(51.3)+3\Delta G^{0}f(-237.1)]-[2\Delta G^{0}f(-110.9)+1\Delta G^{0}f(87.6)]](/tpl/images/0147/7522/2150b.png)