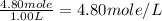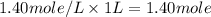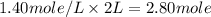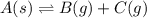Chemistry, 31.07.2019 21:20 adaakbulut9
A4.80 mol sample of solid a was placed in a sealed 1.00 l container and allowed to decompose into gaseous b and c. the concentration of b steadily increased until it reaches 1.40 m, where it remained constant. a(s)↽−−⇀b(g)+c(g) then, the container volume was doubled and equilibrium was re‑established. how many moles of a remain

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
A4.80 mol sample of solid a was placed in a sealed 1.00 l container and allowed to decompose into ga...
Questions

Mathematics, 13.05.2021 23:40

Mathematics, 13.05.2021 23:40




Mathematics, 13.05.2021 23:40




History, 13.05.2021 23:40

Mathematics, 13.05.2021 23:40


Mathematics, 13.05.2021 23:40



Mathematics, 13.05.2021 23:40


World Languages, 13.05.2021 23:40

Mathematics, 13.05.2021 23:40