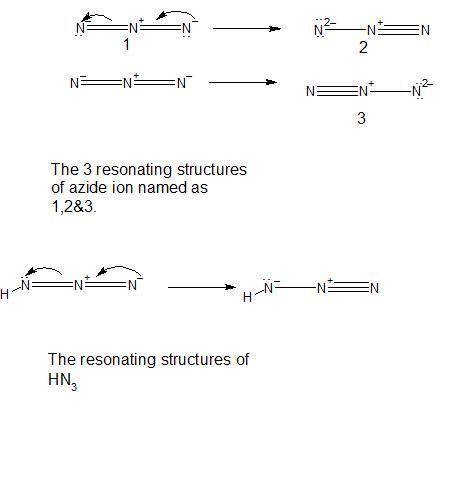
Be sure to answer all parts. pure hn3 (atom sequence hnnn) is explosive. in aqueous solution, it is a weak acid that yields the azide ion, n3−. draw one resonance structure for n3− and one resonance structure for hn3. include all lone pair electrons and nonzero formal charges in your structures.

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1

Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
You know the right answer?
Be sure to answer all parts. pure hn3 (atom sequence hnnn) is explosive. in aqueous solution, it is...
Questions


Mathematics, 04.11.2020 23:30

Mathematics, 04.11.2020 23:30



Computers and Technology, 04.11.2020 23:30



Mathematics, 04.11.2020 23:30


History, 04.11.2020 23:30




English, 04.11.2020 23:30



Mathematics, 04.11.2020 23:30

Mathematics, 04.11.2020 23:30